CTIM-76 Program
CTIM-76: Claudin 6 x CD3 Bispecific Antibody
A novel therapeutic target
There is growing interest in applying antibody modalities including bispecifics, antibody-drug conjugates, and CAR-T to solid tumors. However, identifying appropriate tumor-specific targets that avoid adverse effects in healthy tissue has been challenging. The tight junction protein Claudin 6 (CLDN6) is a validated therapeutic target for many solid tumor types, including ovarian, endometrial, testicular, and gastric. It is differentially expressed on cancer cells with no or limited expression in normal, healthy tissue. It is estimated that there are 70,000 patients with CLDN6-positive metastatic solid tumors in the United States, and no approved targeted treatment options exist.
Overcoming CLDN6 selectivity challenge
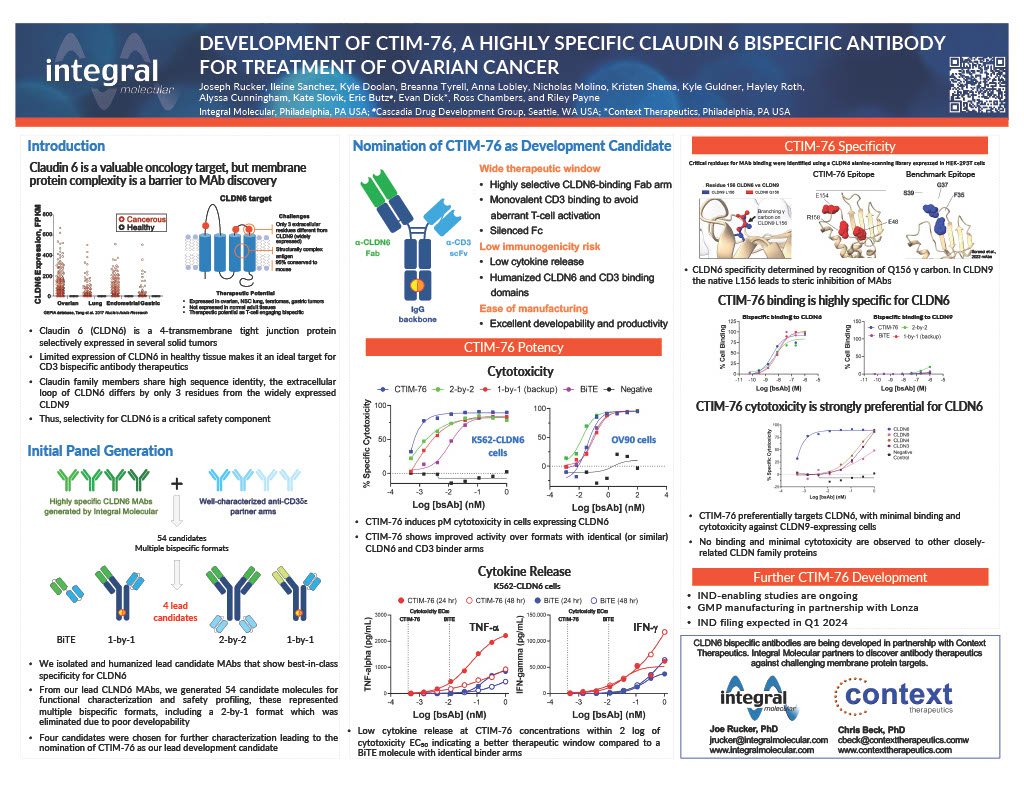
Despite being an attractive target, therapeutic monoclonal antibodies (MAbs) targeting CLDN6 are difficult to discover due to an abundance of closely related family members and an absolute need for high specificity. There are 27 human CLDN family members, and most are broadly expressed and highly conserved. The extracellular region of CLDN6 closely resembles the widely expressed CLDN9 (3 amino acids different). The few CLDN6 MAbs in clinical development have demonstrated significant binding to other CLDN family members and most have now been halted from development. Using Integral Molecular’s MPS antibody discovery platform, we have been able to isolate and optimize rare antibodies against CLDN6 that do not cross-react with other CLDN family members in protein binding assays.
CTIM-76
CTIM-76 is a CLDN6 x CD3 bispecific antibody that incorporates a highly selective CLDN6 binding arm and a CD3 binding single-chain Fv domain in an IgG format with a silenced Fc that is designed to be functionally monovalent to avoid aberrant T-cell activation and to enhance the safety profile. Research has demonstrated that CTIM-76 is potent with specific lysis of CLDN6+ cancer cells over normal cells and can activate cytotoxic T cells without concomitant activation of free cytokines – critical determinants of immunotherapy safety and activity. Preclinical research suggests the potential for convenient dosing with low immunogenicity risk and manufacturing can be scalable to address the significant number of patients who are potentially eligible for CTIM-76 therapy.
Additionally, preclinical studies illustrate the potential of CTIM-76 to treat CLDN6-positive tumors including:
CTIM-76 was shown to have high potency and target selectivity in both binding and cytotoxicity assays.
In in vivo xenograft experiments, CTIM-76 induced dose-proportional tumor regressions and was well tolerated.
In IND-enabling toxicology studies, CTIM-76 was well tolerated, and a potential first-in-human dose was identified.
Clones of clinical-stage CLDN6-targeted molecules were generated for benchmarking purposes. In comparison studies, CTIM-76 activity was retained across a range of cell lines expressing with low through high CLDN6. Additionally, CTIM-76 demonstrated ten-fold higher potency in in vitro cytotoxicity and cytokine activation.
Context Therapeutics dosed the first patient in its Phase 1 clinical trial evaluating CTIM-76 in January 2025.